Visicol (Sodium Phosphate Monobasic Monohydrate, Sodium Phosphate Dibasic Anhydrous): Uses, Dosage, Side Effects, Interactions, Warning

Dulcolax tablets Sodium picosulfate is another cathartic with osmotic... | Download Scientific Diagram

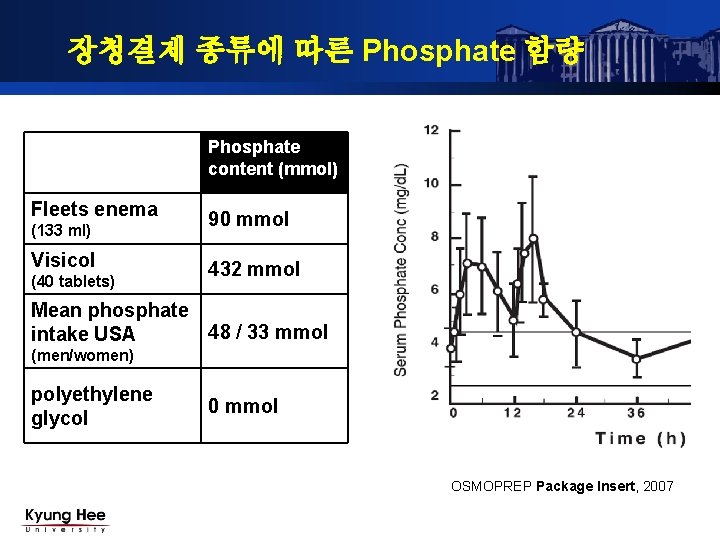
NDC 65649-701 Osmoprep Sodium Phosphate, Monobasic, Monohydrate, Sodium Phosphate, Dibasic Anhydrous
Sodium phosphate products available in the US: OsmoPrep and Visicol.... | Download Scientific Diagram
These highlights do not include all the information needed to use OsmoPrep safely and effectively. See full prescribing informa

Visicol (Sodium Phosphate Monobasic Monohydrate, Sodium Phosphate Dibasic Anhydrous): Uses, Dosage, Side Effects, Interactions, Warning